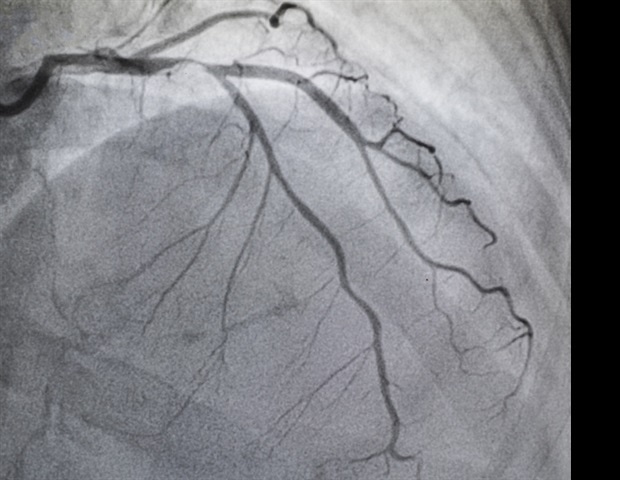
New insights from the TWILIGHT trial showed that ticagrelor monotherapy after three months of ticagrelor plus aspirin was associated with similar rates of recurrent coronary revascularization, major adverse cardiac and cerebrovascular events (MACCE) and a lower risk of net adverse clinical events (NACE) compared with duel antiplatelet therapy (DAPT). The results from the randomized control trial of more than 7,000 patients were presented today as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 Scientific Sessions.
Despite advances in percutaneous coronary intervention (PCI) techniques, devices and secondary prevention, repeat revascularization remains a concern and is associated with increased morbidity, hospitalization and healthcare costs.
In the TWILIGHT trial, high-risk patients who were event-free and adherent to a ticagrelor-based DAPT for three months after PCI were randomized to ticagrelor plus aspirin or ticagrelor plus placebo for 12 additional months. The primary endpoint was repeat revascularization due to recurrent or persistent symptomatic myocardial ischemia. Secondary endpoints included target lesion revascularization (TLR), target vessel revascularization (TVR) and major adverse cardiac and cerebrovascular events (MACCE) and net adverse clinical events (NACE). All endpoints were adjudicated and assessed at 12 months after randomization in the per-protocol population.
Among 7,039 patients, ticagrelor monotherapy and ticagrelor plus aspirin were associated with a similar risk of repeat revascularization (7.1% vs 6.6%, HR 1.09, 95% CI 0.90-1.30), TLR, TVR and MACCE, while NACE was lower with ticagrelor monotherapy. The results also showed that clinically-driven revascularization was associated with an excess risk for subsequent death, myocardial infarction or stroke (aHR 2.92, 95% CI 1.82 – 4.67).
In high-risk PCI patients, it’s critical to lessen the occurrence of repeat revascularization. The findings presented today from the TWILIGHT trial provide a greater understanding of the impact of DAPT and ticagrelor monotherapy following PCI for the clinical community.”
Usman Baber, M.D., Associate Professor of Medicine at the University of Oklahoma Health Sciences Center, and lead author of the study